Rayovac AA Batteries Energy Test
Experiment
Summary: How much energy is in two Rayovac AA alkaline cells and how long will they light a Mini-Maglite flashlight with a cluster of 3 White LEDs? To determine this we recorded the circuit current and both the loaded and unloaded battery voltages at the start and at the end of this test. We considered the end to be the point that the light had dimmed noticeably to the human eye. We started without a lot of empirical seed data, but partially through the experiment we obtained spec sheet data for AA alkaline batteries from the Rayovac web site. We used that data in conjunction with our observations to draw our conclusions. These were that we obtained an approximate 12.5 hour run time and could estimate approximately 1914 mAh of energy per Rayovac alkaline 1.5 V cell.
Materials
- (1) Mini-Maglite - 1980s vintage (2 AA Cell variety) with White LEDs (cluster of 3)
- (2) 1.5V Rayovac Alkaline Primary (Non-rechargeable) cells (new - expiration date Dec 2014)
- (1) Micronta multimeter and various clips and wires for circuit testing.
Procedure
- Dismantle the flashlight
- Insert wires into the tiny holes that the LED assembly plugs into
- Hook circuit together to test current draw of the three LEDs
- Re-wire the circuit to test the voltage drawn while bulbs were off
- Re-connect the lights into the circuit and test the loaded voltage
- Calculate resistance, power, and make some predictions
- Watch the light for noticeable dimming around predicted time. When LEDs dim, take current, loaded and unloaded voltage measurements
Observations
Measured Values |
||
| Current drawn | .174 | amps |
| Voltage unloaded | 3.06 | volts |
| Voltage loaded | 2.88 | volts |
| Calculated voltage drop | 0.18 | Volts |
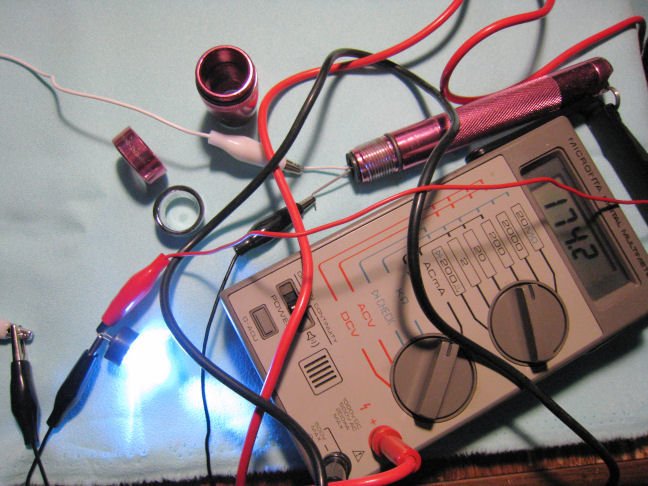
This first image shows the digital Micronta multimeter set to read DC current. The meter was hooked into the circuit while the LEDs were connected in series with the two 1.5 volt alkaline cells. The current was 174 mA.
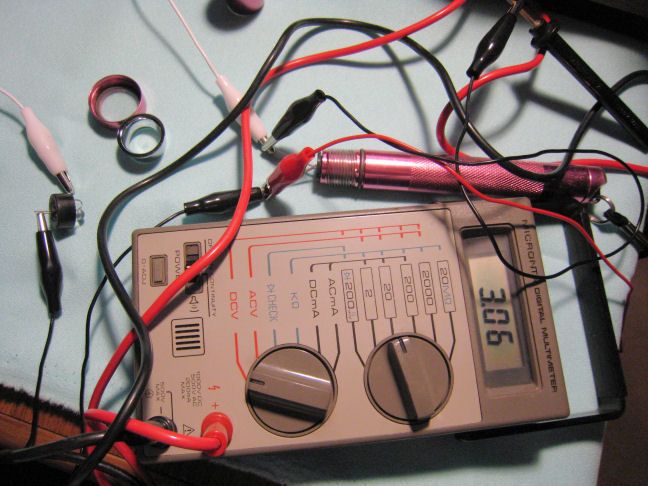
Then the circuit was taken apart and the meter pulled out from in-line. With the circuit open and the LEDs not illuminated the voltage read across the battery terminals was 3.06 volts.
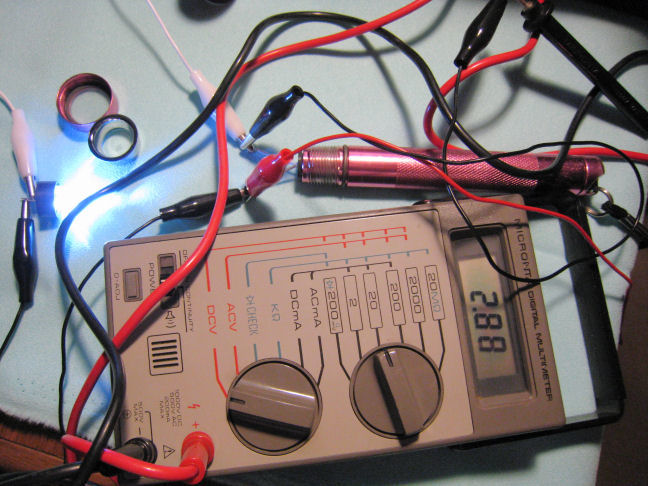
Finally, the LEDs were wired into the circuit once again and the voltage was again measured at the battery terminals. This time there was a lower voltage measured, 2.88 volts.
The difference between the loaded and unloaded voltages was the amount of voltage dropped across the internal resistance of the AA cells.
Calculations
With these measurements in hand, it was now possible to do a few calculations:Formula for load resistance of LEDs:
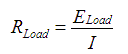
When calculated:
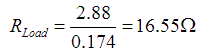
Formula for internal resistance of AA Cells:
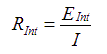
When calculated:
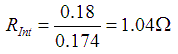
Formula for power draw rate of LEDs:
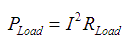
When calculated:
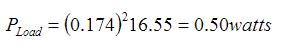
I advanced an estimate of 2000 mAh per cell as what I thought to be a reasonable estimate of the energy in an AA cell. I didn't pick the number entirely from the air. I had recently purchased several rechargeable Duracell alkaline cells and they had the number "2650 mAh" painted on each cell. Not really knowing if these non-rechargeable cells would be more or less, I picked a number that would be relatively easy to calculate with.
Each battery was in the circuit so each had 174 mA running through it. When 2000 mAh is divided by 174mA the answer is approximately 11.494 hours. This was the initial estimate I calculated for how long the LEDs would glow at a reasonably bright level. And 11.494 hours at .50 watts is approximately 5.75 watt-hours. That was the initial estimate for the total energy in the two AA cells available for use in the load.
I started this experiment simply with the intent of finding out how long the two AA batteries would run my flashlight for. I did not plan to capture this much detail, but as I went along I figured this might be something I could put on the web. Before I had proceeded too far though, I went to the Rayovac web site. There they had an Application Notes & Product Data Sheet for all of their carbon zinc and alkaline cells. I was able to find the data shown in the table below on their spec sheet.
Rayovac Specs for Alkaline AA Battery |
|||||||
Estimated Life (Hrs) at 70°F - Cutoff voltages |
|||||||
| Use Pattern | Load Ohms | Current (mA) at 1.2 V | 1.2 V | 1.1 V | 1.0 V | 0.9 V | Approx mAh Capacity to 0.9 V |
| 4 Hrs/Day | 43 | 28 | 59 | 69 | 80 | 88 | 2535 |
| 1 Hr/Day | 10 | 120 | 10 | 14 | 16 | 19 | 2216 |
| 1 Hr/Day | 3.9 | 308 | 2 | 4 | 6 | 7 | 1907 |
| Continuous | 3.9 | 308 | 2 | 4 | 5 | 6 | 1785 |
OK, it looks pretty good. But they are using different loads,
employing different usage patterns, and have a different method of measuring than that used
for this experiment. Had I found this information earlier, I would have structured the experiment
somewhat differently. Regardless, the table provides a lot of insight into how the
cells perform and should allow a little closer estimate of the cells' energy content to be made.
To start with, let's figure the current for our circuit at 1.2V, Rayovac's benchmark. We will work with the data we have already measured and attempt to standardize it to Rayovac's numbers.
To get the current we will work with the resistance we already calculated for the load, 16.55 ohms. We have two AA cells - so we need to use 2.4 V instead of the 3.0 V we originally used.
Circuit Current Formula
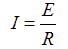
Circuit Current at 2.4 Volts
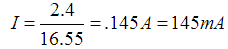
The continuous use example Rayovac shows is a 3.9 ohm load on one cell. Our experiment is an approximately 16 ohm load on 2 cells, The trick here is to use their numbers to let us estimate what mAh capacity we can hypothesize for our case, which is not represented here.
Let's break it down a little: If we had only one cell and it was being run through 8 ohms resistance we could at least place that case in Rayovac's table. It would fall between the 3.9 ohm 1 hr/Day use and the 10 ohm 1 hr/Day use lines. Our current at 1.2 V per cell would be 145mA. There appears to be a somewhat linear relationship between hours of life to 0.9V and the resistance of the loads. This would imply something on the order of 8/10 * 19 hours or about 15 hours for our load. We can also see that there is not a radical difference between drawing the cell down through 3.9 ohms whether it is continuous usage or at the rate of an hour per day. Perhaps 5% fewer amps are available if the lights are used continuously. So if we take 95% of 15, we get about 14 hours.
To start with, let's figure the current for our circuit at 1.2V, Rayovac's benchmark. We will work with the data we have already measured and attempt to standardize it to Rayovac's numbers.
Comment: One important fact this table highlights and that my initial estimates overlooked is that there is
a drop in voltage as the experiment proceeds, therefore, a drop-off in current draw over the usage period.
This could actually extend the period the LEDs might glow.
To get the current we will work with the resistance we already calculated for the load, 16.55 ohms. We have two AA cells - so we need to use 2.4 V instead of the 3.0 V we originally used.
Circuit Current Formula
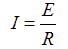
Circuit Current at 2.4 Volts
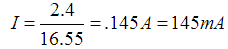
The continuous use example Rayovac shows is a 3.9 ohm load on one cell. Our experiment is an approximately 16 ohm load on 2 cells, The trick here is to use their numbers to let us estimate what mAh capacity we can hypothesize for our case, which is not represented here.
Let's break it down a little: If we had only one cell and it was being run through 8 ohms resistance we could at least place that case in Rayovac's table. It would fall between the 3.9 ohm 1 hr/Day use and the 10 ohm 1 hr/Day use lines. Our current at 1.2 V per cell would be 145mA. There appears to be a somewhat linear relationship between hours of life to 0.9V and the resistance of the loads. This would imply something on the order of 8/10 * 19 hours or about 15 hours for our load. We can also see that there is not a radical difference between drawing the cell down through 3.9 ohms whether it is continuous usage or at the rate of an hour per day. Perhaps 5% fewer amps are available if the lights are used continuously. So if we take 95% of 15, we get about 14 hours.
Now lets try to calculate approximately where in time the
cutoff voltage points might fall. To make it simple, we will use 95% of
80% of the hours shown in the 10 ohm example above.
Hours to Cutoff Voltages |
||||
| 1.2 V | 1.1 V | 1.0 V | 0.9 V | |
| 7.6 Hr | 10.64 Hr | 12.16 Hr | 14.44 Hr | |
Knowing that, we can calculate the mAh used over each
period to come up with the estimated total current
that Rayovac's numbers might imply for our case.
Estimated mAh Used Each Period |
|||||
| Cutoff Voltage Range | 1.5->1.2V | 1.2->1.1V | 1.1->1.0V | 1.0->0.9V | |
| mA usage rate-this period | 169 mA | 144 mA | 131 mA | 119 mA | |
| Estimated Hours at this rate | 7.6 Hr | 3.04 Hr | 1.52 Hr | 2.28 Hr | |
| mAh used | 1283 mAh | 437 mAh | 200 mAh | 271 mAh | |
Total mAh estimated available 2191.
Total mAh estimated out of a single cell is estimated to be 2191 based on the Rayovac calculations.
Total mAh estimated out of a single cell is estimated to be 2191 based on the Rayovac calculations.
Results
Start of Experiment: Light turned on at 9:15 AM Thursday August 16, 2007.End of Experiment: Light was dimmed noticeably by 10:15 PM. It may have started getting dimmer a little sooner (maybe 20 minutes or so) but this is when I actually noticed it being significantly dimmer.
Measured Values at End |
||
| Current drawn | 0.0325 | amps |
| Voltage unloaded | 1.6 | volts |
| Voltage loaded | 0.335 | volts |
| Calculated voltage drop | 1.265 | Volts |
| Calculated running time | 13.0 | Hours |
Calculate the final resistances and power consumption of the circuit
Formula for load resistance of LEDs:
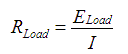
When calculated:
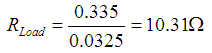
Formula for internal resistance of AA Cells:
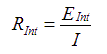
When calculated:
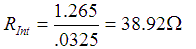
Formula for power draw rate of LEDs:
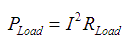
When calculated:
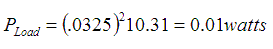
Conclusions
Comment: This is about 3 times the current draw I expected. I thought LEDs took about 20 mA. Depending on the internal hookup arrangement, I would have expected anywhere from 20 - 60 mA draw. Initial speculation: Maybe there is a low value shunt resistor in parallel? Answer - LEDs come in many different varieties. These are quite likely to be High Luminosity LEDs. Those take more current than the LEDs I am accustomed to seeing. These take perhaps 60 mA each. (e.g. 174mA / 3 = 58mA) I found a data sheet belonging to Philips while doing research on this point. It shows a type of High Luminosity LED that they manufacture that takes about 60 mA. They have others that I saw that drew 350 mA or more. So, my initial thought that "all LEDs take around 20 mA" was way off-base.Comment: Was Rayovac's voltage measured loaded or unloaded? If we look at the final voltages, we saw we measured 0.335 Volts total when the circuit was loaded and 1.6 Volts when it was unloaded. 1.6V / 2 gives 0.8V which is a reasonable approximation to the 0.9V Rayovac used in their table. I don't have the answer to this question. Perhaps it is worthy of further study.
Comment: Without initially taking into consideration that interim measurements of current and voltage would have been very useful, we now have only two ways of calculating the energy content of these two AA cells. The first is to create a graph with a straight line decrease in amperage from time zero to time 13 hours and figure the area under the curve as the total mAh drawn. Below is a graphic of what that would look like:
Straight Line Decline Hypothesis:
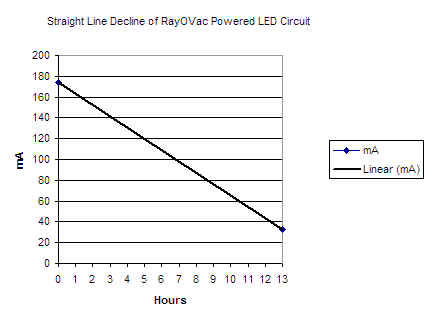
This clearly is incorrect. If we look at this as a right triangle and calculate the area under the curve as 1/2bh we get 1/2 * 13 * .174 = .920 mAh. Easy to calculate, but this seems low by a factor of two. Straight line decline is not how the cells function physically.
The second way is to create an approximate curve based on the estimated draw down we hypothesized based on Rayovac's AA cell specs. Again, we would calculate the area under the curve as being the mAh drawn.
Rayovac Model Decline Hypothesis:
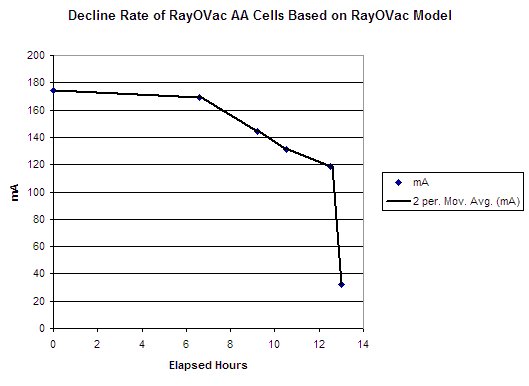
This is much better. If we integrate the area under the curve by taking the average current draw over each of the five intervals we have identified we end up with a much more reasonable, and believable number.
The numbers were calculated by taking the values in the "Hours To Cutoff Voltages" table (above) and then by prorata adjusting the hours down from 14.44 total to 12.5 total. I stopped at 12.5 because at 12.5 hours I still had not seen a marked drop-off in the brightness of the LEDs. I am assuming that the voltage delivered at that time was near 0.9 volts per cell. Then, for the last 30 minutes, once again I make the assumption that the voltage radically dropped off to end up at the level I recorded at the end. (I know that secondary (rechargeable NiMh) cells have this kind of decline pattern. I am not sure that this is totally the case for these primary cells, but for wont of anything more accurate for now, we'll go with it. This next table will show the numbers thus derived:
Hours to Cutoff Voltages |
||||
| 1.2 V | 1.1 V | 1.0 V | 0.9 V | |
| 6.6 Hr | 9.2 Hr | 10.5 Hr | 12.5 Hr | |
And once again we can employ the table shown above named "Estimated mAh Used Each Period" and put in the adjusted numbers to arrive at an estimate observed total mAh number.
Estimated mAh Used Each Period - Based on Observations |
||||||
| Cutoff Voltage Range | 1.5->1.2V | 1.2->1.1V | 1.1->1.0V | 1.0->0.9V | 0.9V->0.163V | |
| mA usage rate-this period | 169 mA | 144 mA | 131 mA | 119 mA | 32.5 mA | |
| Estimated Hours at this rate | 6.6 Hr | 2.6 Hr | 1.3 Hr | 2.0 Hr | 0.5 Hr | |
| mAh used | 1112 mAh | 379 mAh | 172 mAh | 235 mAh | 16 mAh | |
Figuring it this way we arrive at a total of 1914 mAh per Rayovac AA Alkaline cell.
Learnings
Hmm... there are a lot of assumptions here. But again, starting out without being fully aware of the decline curve that these cells have and without having thought to look at the Rayovac web site before we started to see if they had any specs, we obtained about the level of accuracy possible.The correct way to do this experiment would be to have a continuous monitoring of the voltage and current of the circuit, rather than simply a beginning and ending reading. That way, the pro-forma Rayovac numbers could be more directly tested. A large percentage of the accuracy of the number calculated for the total mAh is derived from the shape of the decline curve. And the best we could do here was to estimate it.
And as I questioned earlier, were the voltage cutoff points Rayovac has listed in their table considered to be with or without the load? I would assume (there's that word again) that the voltages would be unloaded circuit voltages. After all, that is the way the voltage readings are taken for lead-acid batteries.
So, to wrap it up, I was able to determine conclusively that you can run the Mini-Maglite flashlight for about 12 1/2 hours continuously on a set of two AA Alkaline cells. I was able to estimate, less accurately that I would have liked, that there is somewhere in the neighborhood of 1900-2000 mAh and about 2.4 watt Hours of energy per cell available.